
Oxidative phosphorylation
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص228-229
الجزء والصفحة:
ص228-229
 2025-11-19
2025-11-19
 41
41
Oxidative phosphorylation
The reactions that occur in complexes I, III, and IV are sufficiently exergonic to drive the synthesis of ATP in the process called oxidative phosphorylation:
ADP +Pi− +H+→ATP ∆rG⊕=+31 kJ mol−1
We saw above that the phosphorylation of ADP to ATP can be coupled to the exergonic dephosphorylation of other molecules. Indeed, this is the mechanism by which ATP is synthesized during glycolysis and the citric acid cycle. However, oxidative phosphorylation operates by a different mechanism. The structure of a mitochondrion is shown in Fig. 7.17. The protein complexes associated with the electron transport chain span the inner membrane and phosphorylation takes place in the intermembrane space. The Gibbs energy of the reactions in complexes I, III, and IV is first used to do the work of moving protons across the mitochondrial membrane. The complexes are oriented asymmetrically in the inner membrane so that the protons abstracted from one side of the membrane can be deposited on the other side. For example, the oxidation of NADH by Q in complex I is coupled to the transfer of four protons across the membrane. The coupling of electron transfer and proton pumping in complexes III and IV contribute further to a gradient of proton concentration across the membrane. Then the enzyme H+-ATPase uses the energy stored in the proton gradient to phosphorylate ADP to ATP. Experi ments show that 11 molecules of ATP are made for every three molecules of NADH and one molecule of FADH2 that are oxidized by the respiratory chain. The ATP is then hydrolysed on demand to perform useful biochemical work throughout the cell. The chemiosmotic the oryproposed by Peter Mitchell explains how H+-ATPases syn the size ATP from ADP. The energy stored in a transmembrane proton gradient come from two contributions. First, the difference in activity of H+ ion results in a difference in molar Gibbs energy across the mitochondrial membrane
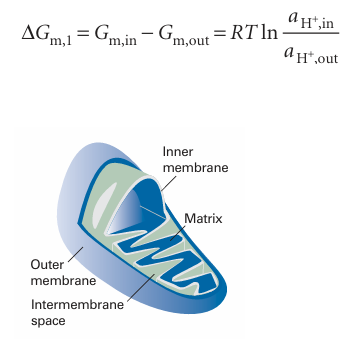
Fig. 7.17 The general features of a typical mitochondrion.
Second, there is a membrane potential difference ∆φ = φin − φout that arises from differences in Coulombic interactions on each side of the membrane. The charge difference across a membrane per mole of H+ ions is NAe, or F, where F = eNA. It follows from Justification 7.3, that the molar Gibbs energy difference is then ∆Gm,2 = F∆φ. Adding this contribution to ∆Gm,1 gives the total Gibbs energy stored by the combi nation of an activity gradient and a membrane potential gradient:
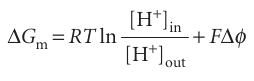
where we have replaced activities by molar concentrations. This equation also pro vides an estimate of the Gibbs energy available for phosphorylation of ADP. After using ln [H+] ≈ ln 10 × log [H+] and substituting ∆pH = pHin − pHout =−log [H+]in + log [H+]out, it follows that
∆Gm=F∆φ−(RTln 10)∆pH
In the mitochondrion, ∆pH ≈−1.4 and ∆φ≈0.14 V, so ∆Gm≈+21.5 kJ mol−1. Because 31 kJ mol−1 is needed for phosphorylation, we conclude that at least 2 mol H+ (and probably more) must flow through the membrane for the phosphorylation of 1 mol ADP.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


