
The respiratory chain
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص227-228
الجزء والصفحة:
ص227-228
 2025-11-19
2025-11-19
 43
43
The respiratory chain
In the exergonic oxidation of glucose 24 electrons are transferred from each C6H12O6 molecule to six O2 molecules. The half-reactions for the oxidation of glucose and the reduction of O2 are
C6H12O6(s) + 6 H2O(l) → 6 CO2(g) + 24 H+(aq) + 24 e− 6 O2(g) + 24 H+(aq) + 24 e− → 12 H2O(l)
The electrons do not flow directly from glucose to O2. We have already seen that, in biological cells, glucose is oxidized to CO2 by NAD+ and FAD during glycolysis and the citric acid cycle:
C6H12O6(s) + 10 NAD+ +2 FAD +4 ADP +4 Pi− + 2 H2O → 6 CO2+10 NADH +2 FADH2+4 ATP +6H+
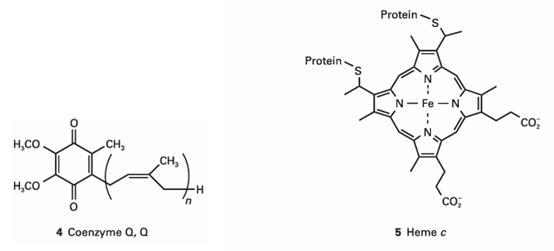
In the respiratory chain, electrons from the powerful reducing agents NADH and FADH2 pass through four membrane-bound protein complexes and two mobile electron carriers before reducing O2 to H2O. We shall see that the electron transfer reactions drive the synthesis of ATP at three of the membrane protein complexes. The respiratory chain begins in complex I (NADH-Q oxidoreductase), where NADH is oxidized by coenzyme Q (Q, 4) in a two-electron reaction:
H++NADH+Q → NAD++QH2 E⊕=+0.42 V, ∆rG⊕=−81 kJ mol−1
Additional Q molecules are reduced by FADH2 in complex II (succinate-Q reductase):
FADH2+Q → FAD+QH2 E⊕=+0.015 V, ∆rG⊕=−2.9 kJ mol−1
Reduced Q migrates to complex III (Q-cytochrome c oxidoreductase), which catalyses the reduction of the protein cytochrome c (Cyt c). Cytochrome c contains the haemcgroup (5), the central iron ion of which can exist in oxidation states +3 and +2. The net reaction catalysed by complex III is
QH2+2 Fe3+ (Cyt c) → →Q+2 Fe2+ (Cyt c) +2 H+
E⊕=+0.15 V, ∆rG⊕=−30 kJ mol−1
Reduced cytochrome c carries electrons from complex III to complex IV (cytochrome c oxidase), where O2 is reduced to H2O:
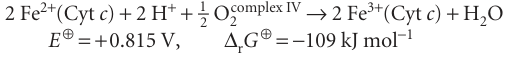
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


