
Oxides of selenium and tellurium
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
412
الجزء والصفحة:
412
 2025-09-25
2025-09-25
 300
300
Oxides of selenium and tellurium
Key points: Selenium and tellurium dioxides are polymorphic; selenium dioxide is thermodynamically less stable than SO2 or TeO2, and selenium trioxide, SeO3, is thermodynamically less stable than SeO2.
The dioxides of Se, Te, and Po can be prepared by direct reaction of the elements. Selenium dioxide is a white solid that sublimes at 315C. It has a polymeric structure in the solid state (21). It is thermodynamically less stable than SO2 or TeO2 and is reduced to selenium on reaction with NH3, N2H4, or aqueous SO2.
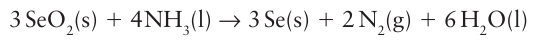
It is used an oxidizing agent in organic chemistry.
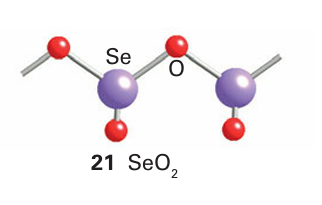
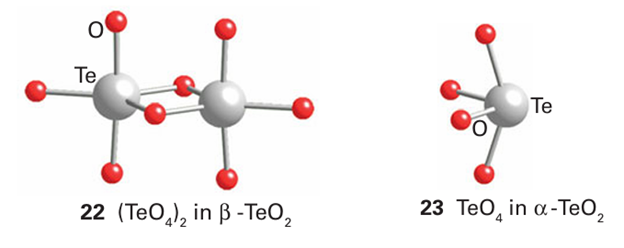
Tellurium dioxide occurs naturally as the mineral tellurite, -TeO2 , which has a layer structure in which TeO4 units form dimers (22). Synthetic -TeO2 consists of similar TeO4 units that share all vertices to form a three-dimensional rutile-like structure (23). Polonium dioxide exists as the yellow form with the fluorite structure and the red tetragonal form.
Selenium trioxide, unlike SO3 or TeO3, is thermodynamically less stable than the dioxide (Table 16.5). It is a white hygroscopic solid that sublimes at 100 ْC and decomposes at 165C. In the solid state the structure is based on Se4O12 tetramers (24).
Table 16.5Standard enthalpies of formation, ∆fHO (kjmol-1), of sulfur, selenium, and tellurium oxides

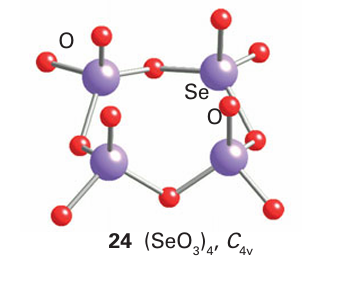
but it is monomeric in the vapour phase. Tellurium trioxide exists as the yellow α-TeO3, which is prepared by dehydration of Te (OH)6, and the more stable ß-TeO3, which is made by heat ing-TeO3 or Te (OH)6 in oxygen.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


