


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 29-12-2021
Date: 6-10-2021
Date: 3-10-2021
|
Isomers and Epimers
Compounds that have the same chemical formula but have different structures are called isomers. For example, fructose, glucose, mannose, and galactose are all isomers of each other, having the same chemical formula, C6H12O6. Carbohydrate isomers that differ in configuration around only one specific carbon atom (with the exception of the carbonyl carbon, see C.1. below) are defined as epimers of each other. For example, glucose and galactose are C-4 epimers because their structures differ only in the position of the –OH (hydroxyl) group at carbon 4. [Note: The carbons in sugars are numbered beginning at the end that contains the carbonyl carbon (that is, the aldehyde or keto group), as shown in Fig. 1.] Glucose and mannose are C-2 epimers. However, because galactose and mannose differ in the position of –OH groups at two carbons (carbons 2 and 4), they are isomers rather than epimers (see Fig. 1).
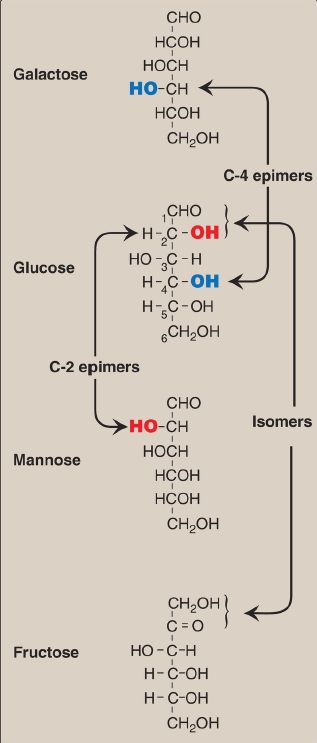
Figure 1: Carbon-2 (C-2) and C-4 epimers and an isomer of glucose.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
عقد جلسة حوارية عن ضحايا جرائم التطرف ضمن فعاليات اليوم الثاني لمؤتمر ذاكرة الألم
|
|
|