


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-7-2019
Date: 27-8-2018
Date: 28-8-2018
|
The alkyl halide reduction described above is one example of a radical substitution reaction. Two other substitution reactions are shown in the diagram below. The first two equations illustrate a useful procedure for reducing alcohols to alkanes, known as "Barton-McCombie deoxygenation". A thionoester, such as a xanthate, is first prepared from the alcohol, and then treated with tributyltin hydride and a radical initiator. Phenylsilane may be substituted for the stannane as a radical carrier. In either case a radical (X• )adds to the C=S function, and the resulting •C(OR)–S–X species fragments to R• and XSC=O. This reduction is particularly useful for 2º-alcohols, as in the second equation. Deoxygenation of 1º-alcohols is often effected by LiAlH4 reduction of tosylate derivatives, a technique that is less satisfactory for 2º-alcohols.
The second equation shows an interesting substitution of sulfur for bromine. Here the phenyl radical intermediate bonds to sulfur, followed by homolysis of the tert-butyl substituent.
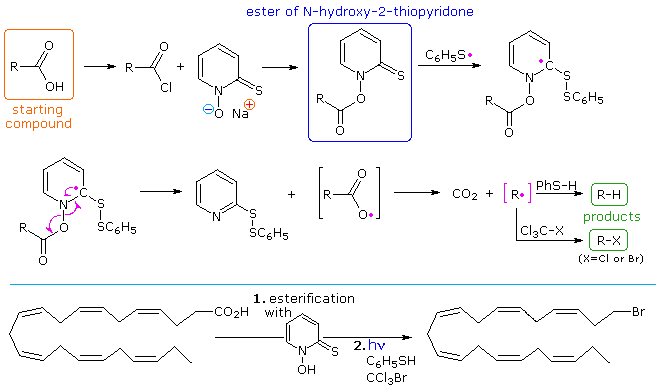
A variation of the Barton-McCombie, called "Barton decarboxylation" will be displayed above. Here advantage is taken of e weak N–O bond to generate a carboxyl radical, which rapidly decarboxylates to an alkyl radical. The N-hydroxy-2-thiopyridone derivative of a carboxylic acid or acid chloride is readily prepared. Photolysis in the presence of a thiol as the hydrogen transfer agent initiates the chain reaction shown above the light blue line. The alkyl radical thus prepared (in pink brackets) may then accept a hydrogen or halogen atom, depending on other reagents chosen for the reaction. The reaction shown below the blue line illustrates the versatility of this procedure.



|
|
|
|
تحذير من "عادة" خلال تنظيف اللسان.. خطيرة على القلب
|
|
|
|
|
|
|
دراسة علمية تحذر من علاقات حب "اصطناعية" ؟!
|
|
|
|
|
|
|
عرض مسرحي بعنوان (رحلة البحث عن التكليف) ضمن فعّاليات حفل التكليف الشرعيّ
|
|
|