
The interhalogens
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص422-423
الجزء والصفحة:
ص422-423
 2025-09-25
2025-09-25
 366
366
The interhalogens
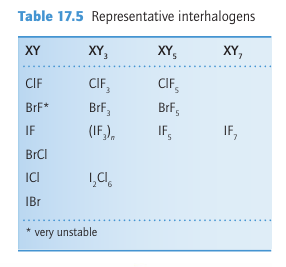
Key point: All the halogens form compounds with other members of the group. The interhalogens are formed between Group 17 elements. The binary interhalogens are molecular compounds with formulas XY, XY3, XY5, and XY7, where the heavier, less electronegative halogen X is the central atom. They also form ternary interhalogens of the type XY2Z and XYZ2, where Z is also a halogen atom. The interhalogens are of special importance as highly reactive intermediates and for providing useful insights into bonding.
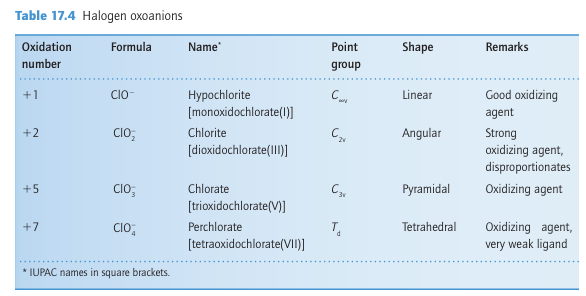
The diatomic interhalogens, XY, have been made for all combinations of the elements, but many of them do not survive for long. All the F interhalogen compounds are exergonic (ΔfGO < 0). The least labile interhalogen is ClF, but ICl and IBr can also be obtained in pure crystalline form. Their physical properties are intermediate between those of their com ponent elements. For example, the deep red -ICl (m.p. 27C, b.p. 97C) is intermediate between yellowish–green Cl2 (m.p. 101C, b.p. 35C) and dark purple I2 (m.p. 114C, b.p. 184C). Photoelectron spectra indicate that the molecular orbital energy levels in the mixed dihalogen molecules lie in the order 3Ϭ2 < 1π4 < 2π4, which is the same as in the homonuclear dihalogen molecules (Fig. 17.2). An interesting historical note is that ICl was discovered before Br2 in the early nineteenth century, and, when later the first samples of the dark red–brown Br2 (m.p. 7C, b.p. 59C) were prepared, they were mistaken for ICl. Most of the higher interhalogens are fluorides (Table 17.5). The only neutral interhalogen with the central atom in a 7-oxidation state is IF7, but the cation ClF6, a compound of Cl (VII), is known. The absence of a neutral ClF7 reflects the destabilizing effect of nonbonding electron repulsions between F atoms (indeed, coordination numbers greater than six are not observed for other p-block central atoms in Period 3). The lack of BrF7 might be rationalized in a similar way, but in addition we shall see later that bromine is reluctant to achieve its maximum oxidation state. This is another manifestation of the alternation effect (Section 9.2c). In this respect, it resembles some other Period 4 p-block elements, notably arsenic and selenium.
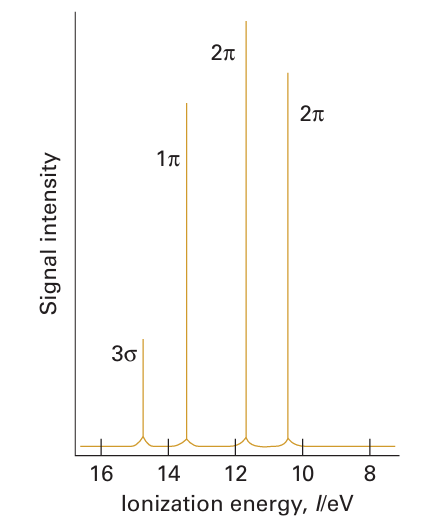
Figure 17.2 Photoelectron spectrum of ICl. The 2π levels give rise to two peaks because of spin-orbit interaction in the positive ion.
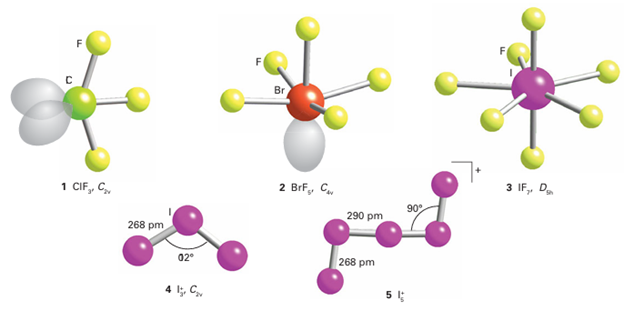
The shapes of interhalogen molecules (1), (2), and (3) are largely in accord with the VSEPR model (Section 2.3). For example, the XY3 compounds (such as ClF3) have five valence electron pairs around the X atom in a trigonal–bipyramidal arrangement. The Y atoms attach to the two axial pairs and one of the three equatorial pairs, and then the two axial bonding pairs move away from the two equatorial lone pairs. As a result, XY3 molecules have a C2v bent T shape. There are some discrepancies: for example, ICl3 is a Cl-bridged dimer. The Lewis structure of XF5 has five bonding pairs and one lone pair on the central X atom and, as expected from the VSEPR model, XF5 molecules are square pyramidal. As already mentioned, the only known XY7 compound is IF7, which is predicted to be pentagonal bipyramidal. The experimental evidence for its actual structure is inconclusive. As with other hypervalent molecules, the bonding in IF7 can be explained without invoking d-orbital participation by adopting a molecular orbital model in which bonding and non-bonding orbitals are occupied but antibonding orbitals are not. Polymeric interhalogens may also be formed and may be cationic or anionic. Examples of cationic polyhalides are I+3 (4) and I5+ (5). Anionic polyhalides are most numerous for io dine. The I-3 ion is the most stable but others with the general formula [(I2)n I-] are formed. Other anionic polyhalides include Cl-3 and BrF-4.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


