


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-12-2015
Date: 26-2-2019
Date: 31-12-2015
|
Single displacement reactions: Kicking out another element
In single displacement reactions, a more active element displaces (kicks out) another less active element from a compound. For example, if you put a piece of zinc metal into a copper (II) sulfate solution, the zinc displaces the copper, as this next equation shows
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
The notation (aq) indicates that the compound is dissolved in water — in an aqueous solution. Because zinc replaces copper in this case, it’s said to be more active. If you instead place a piece of copper in a zinc sulfate solution, nothing will happen.
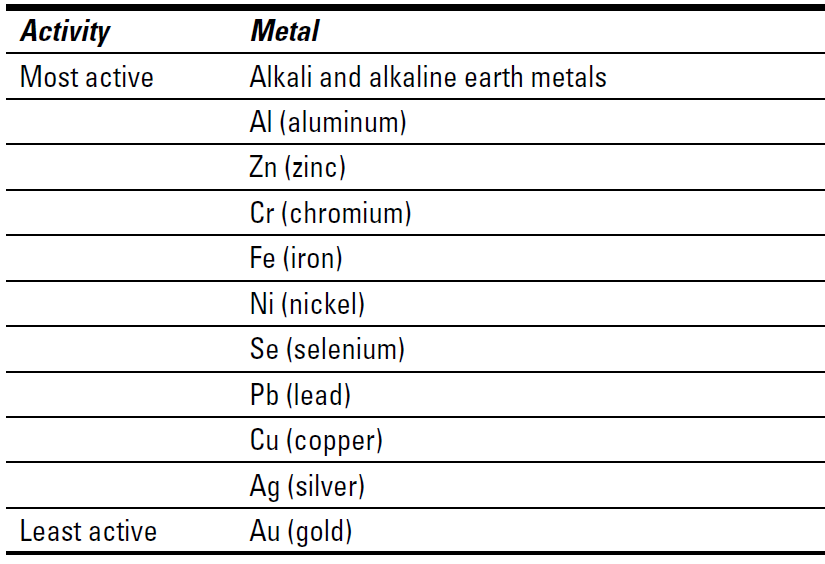
Using the activity series
Table below shows the activity series of some common metals. Notice that because zinc is more active in the table, it will replace copper, just as the earlier equation shows.
Writing ionic and net-ionic equations
Take a look at the reaction between zinc metal and a copper (II) sulfate solution. I’ve written this reaction as a molecular equation, showing all species in the neutral form.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
However, these reactions normally occur in an aqueous (water) solution. When you dissolve the ionically bonded CuSO4 in water, it breaks apart into ions (atoms or groups of atoms that have an electrical charge due to the loss or gain of electrons). The copper ion has a 2+ charge because it lost two electrons. It’s a cation, a positively charged ion. The sulfate ion has a 2– charge because it has two extra electrons. It’s an anion, a negatively charged ion.
Here’s an equation that shows the ions separately. Equations in this form are called ionic equations because they show the reaction and production of ions. Notice that the sulfate ion, SO42–, doesn’t change in the reaction:
Zn(s) + Cu2+ + SO42– → Zn2+ + SO42– + Cu(s)
Ions that don’t change during the reaction and are found on both sides of the equation in an identical form are called spectator ions. Chemists often omit the spectator ions and write the equation showing only those chemical substances that are changed during the reaction. This is called the net-ionic equation:
Zn(s) + Cu2+ → Zn2+ + Cu(s)



|
|
|
|
تحذير من "عادة" خلال تنظيف اللسان.. خطيرة على القلب
|
|
|
|
|
|
|
دراسة علمية تحذر من علاقات حب "اصطناعية" ؟!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تحذّر من خطورة الحرب الثقافية والأخلاقية التي تستهدف المجتمع الإسلاميّ
|
|
|