
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-6-2019
Date: 17-1-2018
Date: 25-7-2020
|
Grouping atoms with the condensed structural formula
We like the Lewis formula because it enables you to show a lot of information without having to write all those little dots. But it, too, is rather bulky. Sometimes chemists (who are, in general, a lazy lot) use condensed structural formulas to show bonding patterns. They may condense the Lewis formula by omitting the nonbonding electrons (dots) and grouping atoms together and/or by omitting certain dashes (covalent bonds). For instance, condensed formulas often group all the hydrogens bonded to a particular carbon atom. Figure 1-1 shows a couple of condensed formulas for C2H4O.
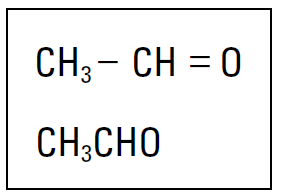
Figure 1-1: Condensed structural formulas for C2H4O.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|