
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Properties of Alcohols and Phenols : The effect of van der Waals forces |
|
|
|
Read More
Date: 12-7-2018
Date: 28-7-2018
Date: 9-9-2019
|
Compare ethane and ethanol:
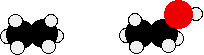
Ethanol is a longer molecule, and the oxygen atom brings with it an extra 8 electrons. Both of these increase the size of the van der Waals dispersion forces, and subsequently the boiling point. A more accurate measurement of the effect of the hydrogen bonding on boiling point would be a comparison of ethanol with propane rather than ethane. The lengths of the two molecules are more similar, and the number of electrons is exactly the same.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|