
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-9-2018
Date: 17-12-2020
Date: 19-12-2020
|
SECOND ORDER REACTIONS
Let us take a second order reaction of the type
2A ⎯⎯→ product
Suppose the initial concentration of A is amoles litre–1
. If after time t, x moles of A has reacted, the concentration of A is (a– x). We know that for such a second order reaction, rate of reaction is proportional to the square of the concentration of the reactant. Thus,

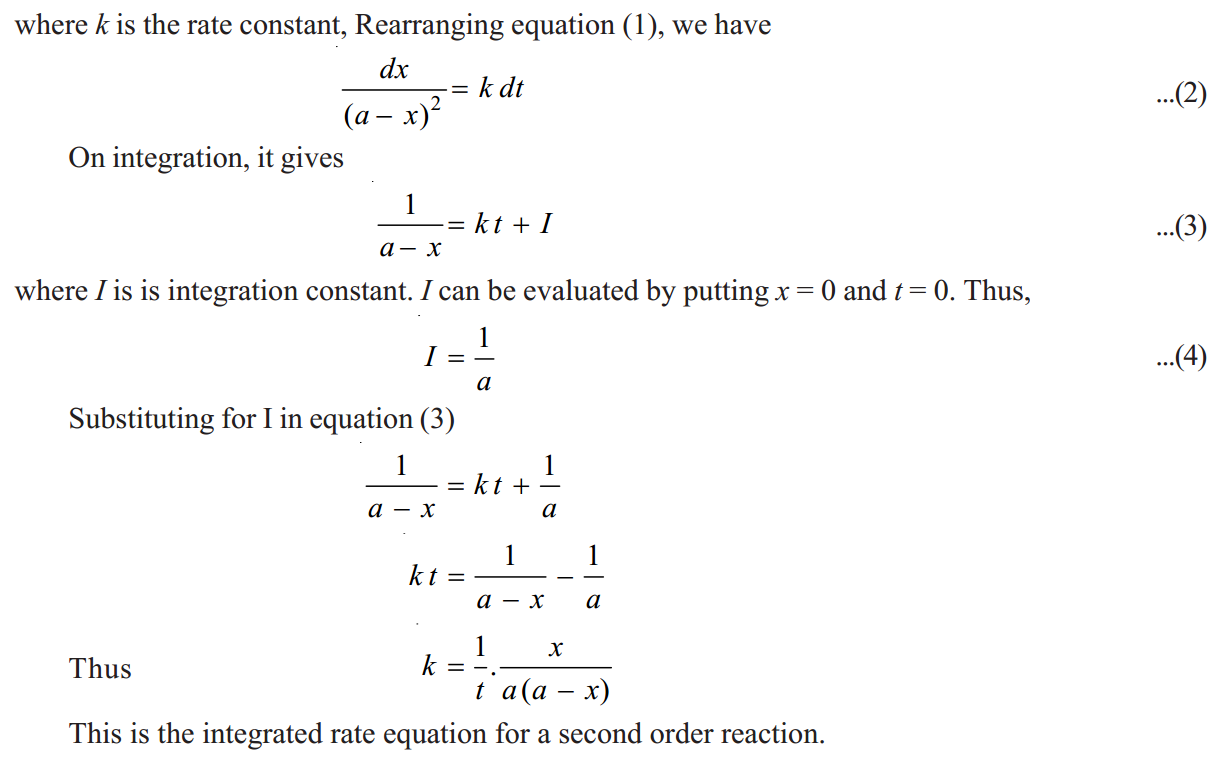



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
الأمانة العامة للعتبة الكاظمية المقدسة تحيي ذكرى أسد الله ورسوله الحمزة "عليه السلام"
|
|
|