
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-9-2018
Date: 25-9-2018
Date: 25-9-2018
|
Temperature Dependence of Rate Constants
Rate constants, like equilibrium constants, are temperature-dependent. Unlike K, however, k virtually always increases with increasing temperature. The temperature-dependence usually obeys the Arrhenius equation,
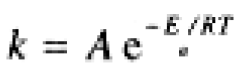 (1.1)
(1.1)
where A is called the frequency factor and Ea is the activation energy. The logarithmic form of the Arrhenius equation,
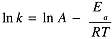
the same form as eq (1.2) for In K, with Ea replacing DH° and In A replacing DS°/R.
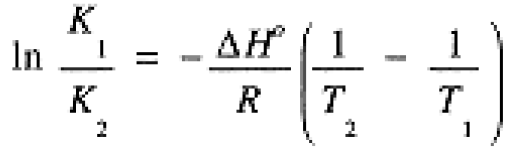 (1.2)
(1.2)
Comparing rate constants measured at two different temperatures, we can derive the analog of Eq. (1.2):
 (1.3)
(1.3)



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
مهرجان تعزيز الذاكرة الأول يشهد افتتاح معرض خاص بجرائم الإبادة الجماعية
|
|
|