
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-4-2017
Date: 26-7-2016
Date: 12-4-2017
|
Molality: Comparing solute to solvent
Molality is another concentration term that involves moles of solute. It isn’t used very much, but you may happen to run across it. Molality (m) is defined as the moles of solute per kilogram of solvent. It’s one of the few concentration units that doesn’t use the total solution’s weight or volume. Mathematically, it looks like this:
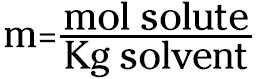
Suppose, for example, you want to dissolve 15.0 grams of NaCl in 50.0 grams of water. You can calculate the molality like this (you must convert the 50.0 grams to kilograms before you use it in the equation):




|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
عوائل الشهداء: العتبة العباسية المقدسة سبّاقة في استذكار شهداء العراق عبر فعالياتها وأنشطتها المختلفة
|
|
|