


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 9-8-2016
Date: 25-8-2016
Date: 10-9-2016
|
van der Waals
A monatomic gas obeys the van der Waals equation
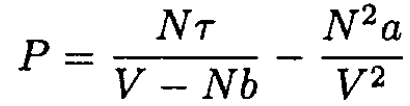
and has a heat capacity CV = 3N/2 in the limit V → ∞.
a) Prove, using thermodynamic identities and the equation of state, that

b) Use the preceding result to determine the entropy of the van der Waals gas, S(τ, V) to within an additive constant.
c) Calculate the internal energy ε(τ, V) to within an additive constant.
d) What is the final temperature when the gas is adiabatically compressed from (V1, τ1) to final volume V2?
e) How much work is done in this compression?
SOLUTION
a) The heat capacity CV is defined as
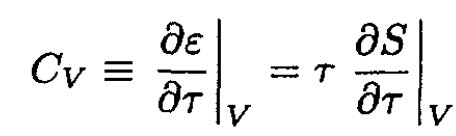 (1)
(1)
By using the Maxwell relation
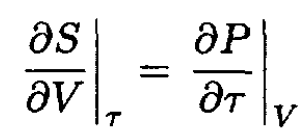 (2)
(2)
we may write
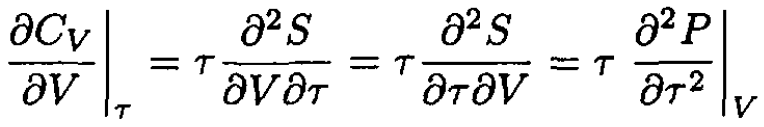 (3)
(3)
Substituting the van der Waals equation of state
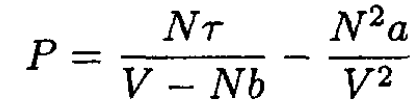 (4)
(4)
into (3) gives
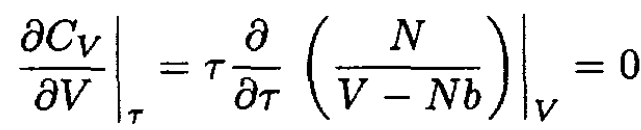 (5)
(5)
b) The entropy S(τ, V) may be computed from
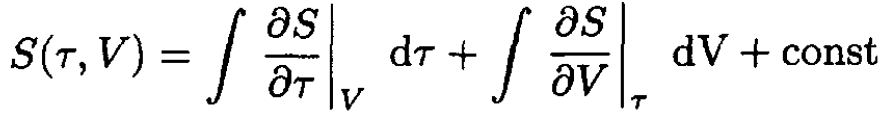 (6)
(6)
We were given that CV = 3N/2 at V → ∞, therefore, again using (2) and (S.4.52.4), we obtain
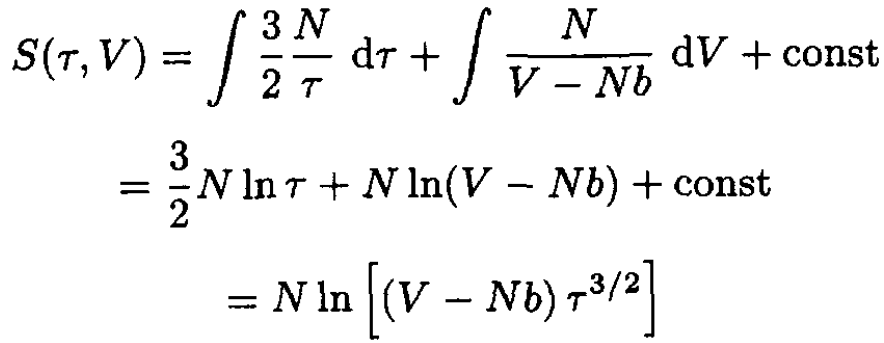 (7)
(7)
c) The internal energy ε(τ, V) may be calculated in the same way from
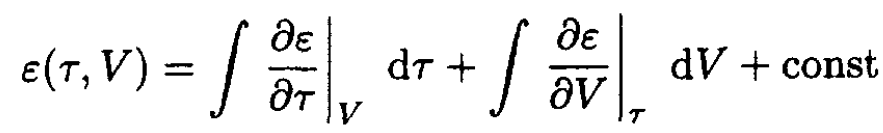 (8)
(8)
Now, from dε = τ dS – P dV, we have
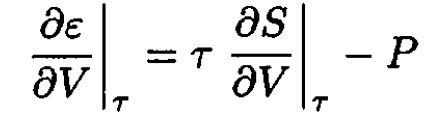 (9)
(9)
and using (4) and (7), we get
 (10)
(10)
So, (8) becomes
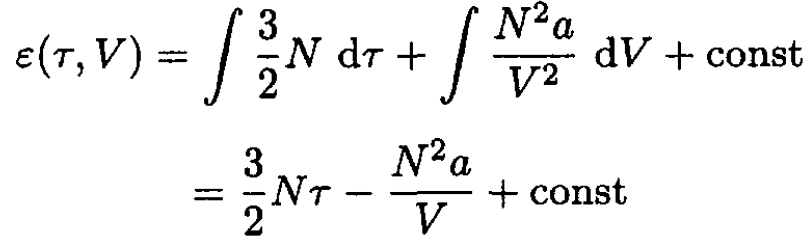 (11)
(11)
d) During adiabatic compression, the entropy is constant, so from (7)
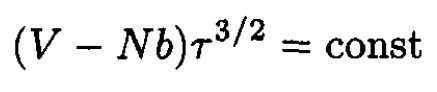 (12)
(12)
and we have
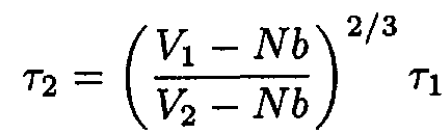 (13)
(13)
e) The work done is given by the change in internal energy ε since the entropy is constant:
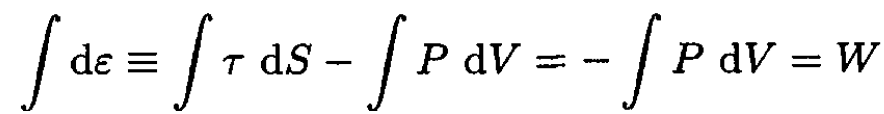 (14)
(14)
From (11), we arrive at
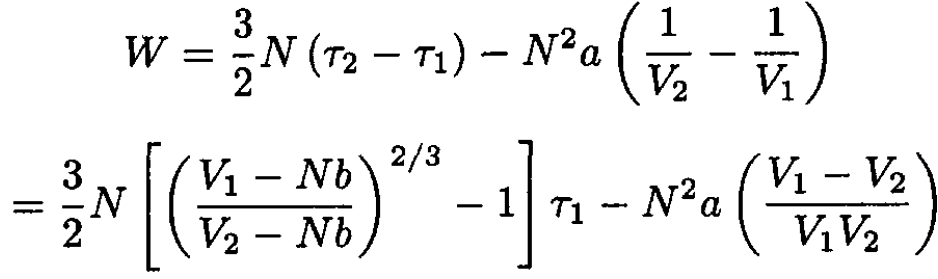 (15)
(15)



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|