
آخر المواضيع المضافة


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 30-8-2016
Date: 29-8-2016
Date: 11-8-2016
|
Phase Transition
The curve separating the liquid and gas phases ends in the critical point Vc, τc, where (∂P/∂V)τ = τc = 0. Using arguments based on thermodynamic stability, determine
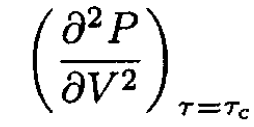
at the critical point.
SOLUTION
For a system at equilibrium with an external reservoir, the Gibbs free energy G = F + P0V is a minimum. Any deviation from equilibrium will raise G:
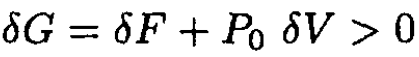 (1)
(1)
where P0 is the pressure of the reservoir. Expanding δF in δV, we have
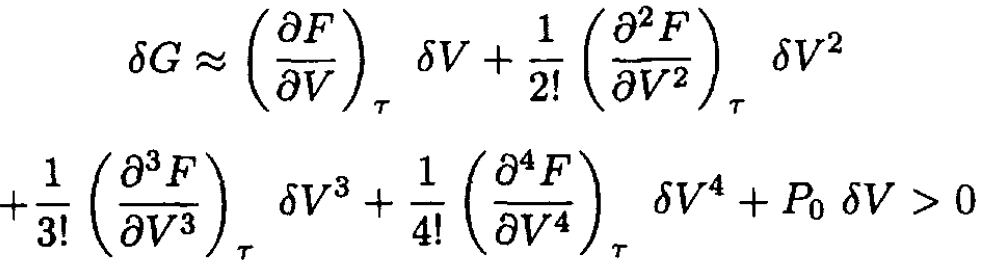 (2)
(2)
Since ∂F/∂V = -P, we may rewrite (2) as
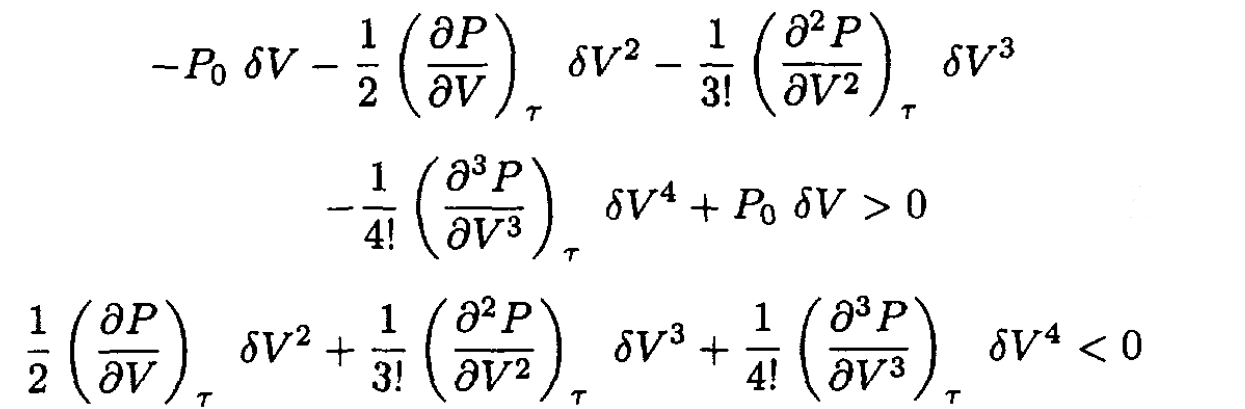 (3)
(3)
At the critical point, ∂P/∂V = 0, so (3) becomes
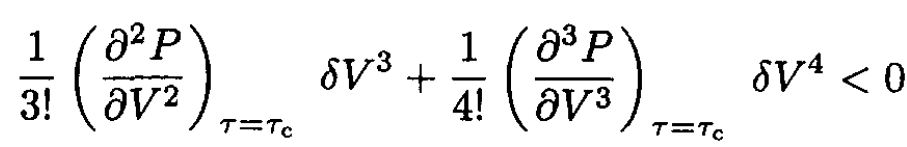 (4)
(4)
For (4) to hold for arbitrary δV, we have
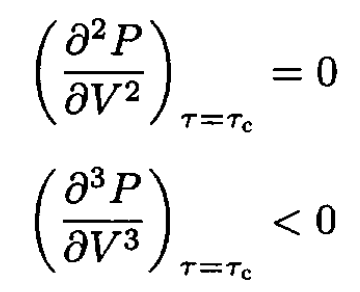 (5)
(5)



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|