
آخر المواضيع المضافة


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 30-12-2016
Date: 9-12-2020
Date: 21-9-2020
|
Isothermal Expansion of an Ideal Gas
If we have one mole of an ideal gas in our cylinder, and keep the temperature constant at a temperature T1 , then the gas will obey the ideal gas equation.
pV = RT1 = constant .........(1)
Thus the equation for the pressure of an ideal gas during an isothermal expansion is
p = constant/V .......(2)
and we see that the pressure decreases as 1/V. This decrease is shown in the pV diagram of Figure (1).
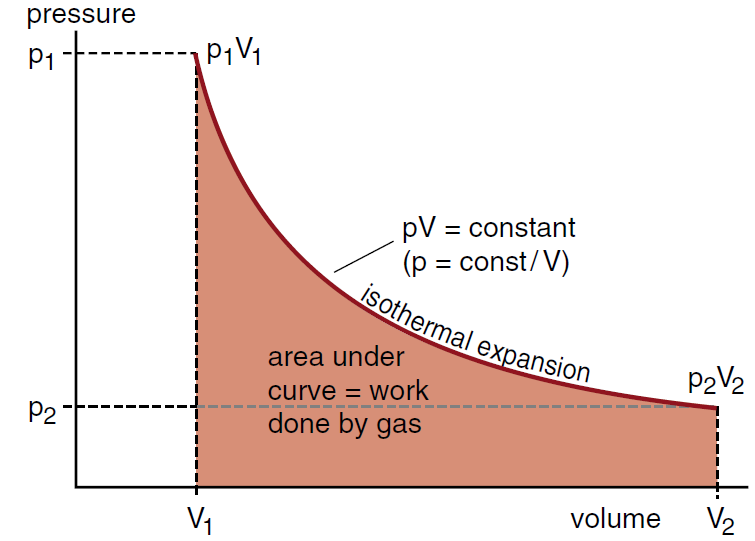
Figure 1: In the isothermal expansion of an ideal gas, we have pV = constant. Thus the pressure decreases as 1/V.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|