


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-1-2020
Date: 28-1-2022
Date: 18-5-2017
|
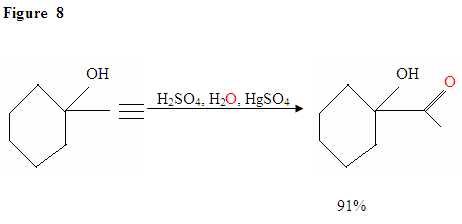
Just as described in Figure 7 the π electrons will attack a proton, forming a carbocation, which then gets attacked by the nucleophilic water molecules. After deprotination, we generate an enol, which then tautomerizes into the ketone form shown.
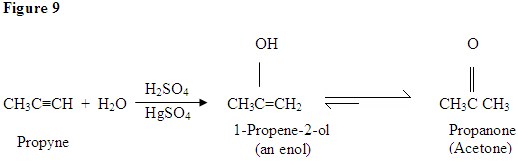
As you can see here, the π electrons of the triple bond are attacking the proton, which forms a covalent bond on the carbon with the most hydrogen substituents. Once the hydrogen is bound you have a carbocation, which gets attacked by the water molecule. Now you have a positive charge on the oxygen which results in a base coming in and deprotinating the molecule. Once deprotinated, you have an enol, which then gets tautomerized.
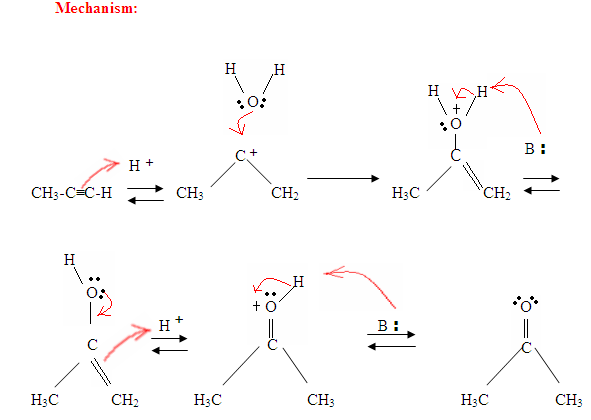
Tautomerism is shown here when the proton gets attacked by the double bond π electrons forming a covalent bond between the carbon and the hydrogen on the less substituted carbon. Electrons from the Oxygen end up moving to the carbon, forming a double bond with carbon and giving itself a positive charge, which then gets attacked by the base. The base deprotinates the Oxygen resulting in the more stable final product at equilibrium, which is a ketone.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|