


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-7-2018
Date: 28-10-2019
Date: 29-7-2018
|
Friedel-Crafts Acylation
The goal of the reaction is the following:
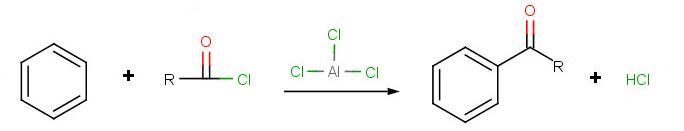.jpg?revision=1)
The very first step involves the formation of the acylium ion which will later react with benzene:
.jpg?revision=1&size=bestfit&width=720&height=114)
The second step involves the attack of the acylium ion on benzene as a new electrophile to form one complex:
.jpg?revision=1)
The third step involves the departure of the proton in order for aromaticity to return to benzene:
.jpg?revision=1)
During the third step, AlCl4 returns to remove a proton from the benzene ring, which enables the ring to return to aromaticity. In doing so, the original AlCl3 is regenerated for use again, along with HCl. Most importantly, we have the first part of the final product of the reaction, which is a ketone. Thie first part of the product is the complex with aluminum chloride as shown:
.jpg?revision=1)
The final step involves the addition of water to liberate the final product as the acylbenzene:
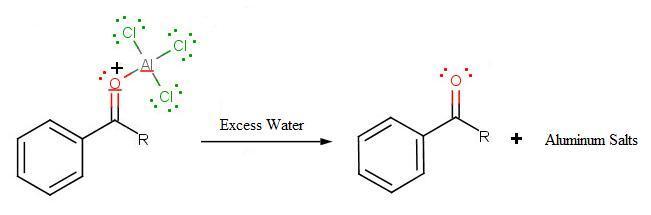.jpg?revision=1)
Because the acylium ion (as was shown in step one) is stabilized by resonance, no rearrangement occurs (Limitation 1). Also, because of of the deactivation of the product, it is no longer susceptible to electrophilic attack and hence, is no longer susceptible to electrophilic attack and hence, no longer goes into further reactions (Limitation 3). However, as not all is perfect, Limitation 2 still prevails where Friedel-Crafts Acylation fails with strong deactivating rings.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|