


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-2-2017
Date: 25-6-2020
Date: 6-7-2017
|
Representing covalent bonds
The overlapping of the electron orbitals and the sharing of an electron pair is represented in Figure 1-1a.
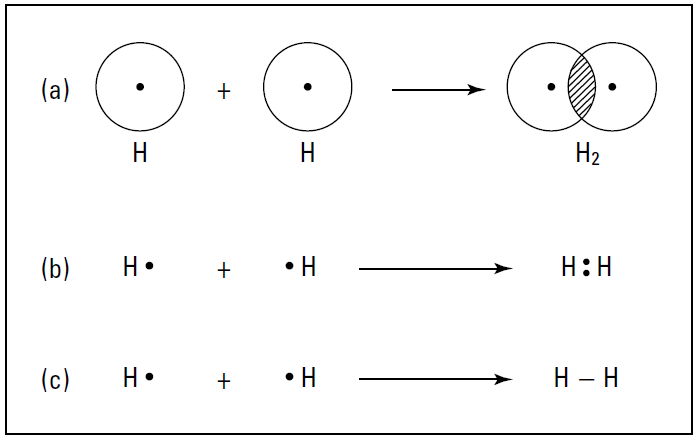
Figure 1-1: The formation of a covalent bond in hydrogen.
Another way to represent this process is through the use of an electron-dot formula. In this type of formula, valence electrons are represented as dots surrounding the atomic symbol, and the shared electrons are shown between the two atoms involved in the covalent bond. Figure 1-1b shows the electrondot formula representations of H2.
Most of the time, I use a slight modification of the electrondot formula called the Lewis structural formula; it’s basically the same as the electron-dot formula, but the shared pair of electrons (the covalent bond) is represented by a dash. Figure 1-1c shows the Lewis structural formula.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|