
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 26-10-2018
Date: 14-10-2018
Date: 22-1-2019
|
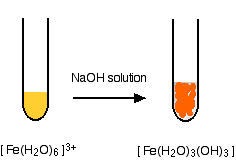
Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the iron ions. When enough hydrogen ions have been removed, you are left with a complex with no charge - a neutral complex. This is insoluble in water and a precipitate is formed.
In the iron(II) case:
In the iron(III) case:
In the test-tube, the color changes are:
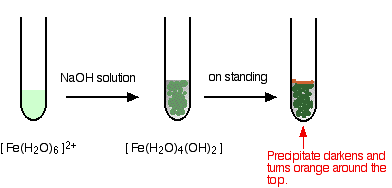
Iron is very easily oxidized under alkaline conditions. Oxygen in the air oxidizes the iron(II) hydroxide precipitate to iron(III) hydroxide especially around the top of the tube. The darkening of the precipitate comes from the same effect.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|