
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-9-2018
Date: 18-12-2020
Date: 25-9-2018
|
Half-life for a Second order Reaction
For the simple second order reaction 2A →Products, the integrated rate equation is
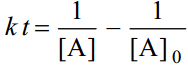
where [A]0 is the initial concentration and [A] is the concentration when time thas elapsed.
When one-half life has elapsed.
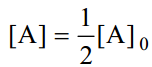
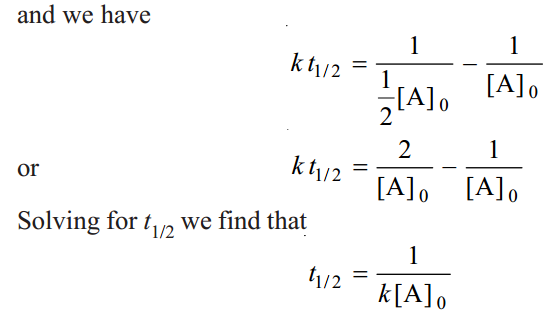
As in case of a first order reaction, half-life for a second order reaction is inversely proportional to rate constant k. While half-life of a first order reaction is independent of initial concentration, half-life of a second order reaction depends on initial concentration. This fact can be used to distinguish between a first order and a second order reaction.
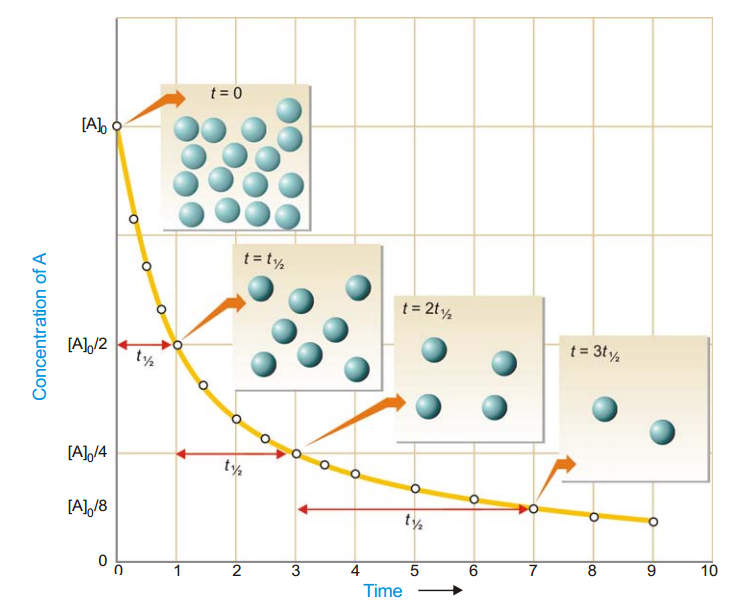



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|